Controlling Cell Fate
Design of scaffolds able to control and steer (stem) cell activity
 Stem cells are a fascinating and promising source to regenerate tissues and organs due to their potential to differentiated into multiple specialized cells. Yet, better control over cell-material interactions is necessary to maintain tissue engineered constructs in time. It is crucial to control stem cell quiescence, proliferation and differentiation in three-dimensional scaffolds while maintaining cells viable in situ.
Stem cells are a fascinating and promising source to regenerate tissues and organs due to their potential to differentiated into multiple specialized cells. Yet, better control over cell-material interactions is necessary to maintain tissue engineered constructs in time. It is crucial to control stem cell quiescence, proliferation and differentiation in three-dimensional scaffolds while maintaining cells viable in situ.
Stem cell activity is controlled by a complex cascade of signals called “niche”, where the extra-cellular matrix (ECM) surrounding the cells play a major role. Designing scaffolds inspired by this cellular niche and its ECM may lead to engineered tissues with instructive properties characterized by enhanced homeostasis, stability and integration with the surrounding milieu.
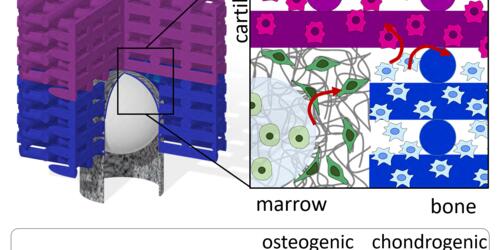
In this research line, we aim at engineering constructs where scaffolds work as stem cell delivery systems actively controlling cell quiescence, proliferation, and differentiation. This challenge will be approached through a biomimetic design inspired by the mesenchymal stem cell niche. Three different scaffolds will be combined to achieve this purpose: (i) a scaffold designed to maintain cell quiescence; (ii) a scaffold designed to promote cell proliferation; and (iii) a scaffold designed to control cell differentiation. To prove the design criteria, the evaluation of stem cell quiescence, proliferation, and differentiation will be assessed for musculoskeletal regenerative therapies.
Researchers involved in this project
David Gomes, Khadija Mulder, Jip Zonderland, Ivan Lorenzo Moldero, Honglin Chen, Sara Neves, Matt Baker, Abhishek Harichandan.
Related publications
Higuera GA, Fernandes H, Spitters TW, van de Peppel J, Aufferman N, Truckenmueller R, Escalante M, Stoop R, van Leeuwen JP, de Boer J, Subramaniam V, Karperien M, van Blitterswijk C, van Boxtel A, Moroni L. Spatiotemporal proliferation of human stromal cells adjusts to nutrient availability and leads to stanniocalcin-1 expression in vitro and in vivo. Biomaterials 2015; Aug;61:190-202.
Download PubblicationCamarero‐Espinosa S, Beeren I, Liu H, Gomes DB, Zonderland J, Lourenço AFH, van Beurden D, Peters M, Koper D, Emans P, Kessler P, Rademakers T, Baker MB, Bouvy N, Moroni L. 3D Niche‐Inspired Scaffolds as A Stem Cell Delivery System for The Regeneration of The Osteochondral Interface. Advanced Materials 2024; 16(1): 2310258.
Download PubblicationChen C, Gigli M, Gualandi C, Truckenmueller R, van Blitterswijk C, Lotti N, Munari A, Focarete ML, Moroni L. Tailoring chemical and physical properties of fibrous scaffolds from block copolyesters containing ether and thio-ether linkages for skeletal differentiation of human mesenchymal stromal cells. Biomaterials 2016 76: 261-272.
Download PubblicationGunnewiek MK, Di Luca A, Bollemaat HZ, van Blitterswijk CA, Vancso GJ, Moroni L, Benetti EM. Creeping Proteins in Microporous Structures: Polymer Brush-Assisted Fabrication of 3D Gradients for Tissue Engineering. Adv Healthc Mater 2015, Jun 3;4(8):1169-74.
Download Pubblication-
Higuera GA, van Boxtel A, van Blitterswijk CA, Moroni L. The physics of tissue formation with mesenchymal stem cells. Trends in Biotechnology 2012; 30(11): 583-590.
Download Pubblication Moroni L, Habibovic P, Mooney DJ, van Blitterswijk CA. Functional tissue engineering through biofunctional macromolecules and surface design. MRS bulletin 2010; 35(8):584:590.
Download PubblicationTerraciano V, Hwang N, Moroni L, Park HB, Zhang Z, Mizrahi J, Seliktar D, Elisseeff J. Differential Response of Adult and Embryonic Mesenchymal Progenitor Cells to Mechanical Compression in Hydrogels. Stem Cells 2007; 25(11): 2730-8.
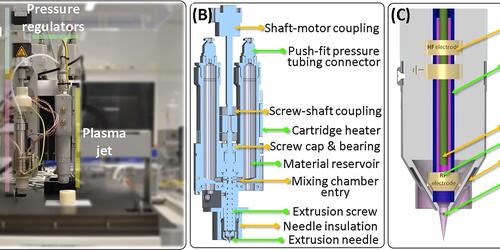
Download PubblicationChakraborty J, Fernandez-Perez J, Takhsha Ghahfarokhi M, van Kampen KA, ten Brink T, Ramis J, Kalogeropoulou M, Cabassi R, de Julián Fernández C, Albertin F, Mota C, Ghosh S, Moroni L. Development of 4D-bioprinted shape-morphing magnetic constructs for cartilage regeneration using a silk fibroin-gelatin bioink. Cell Reports Physical Science 2024; 2(6)
Download Pubblication